 The Infinium Omni series receives new FDA 510K
The Infinium Omni series receives new FDA 510K
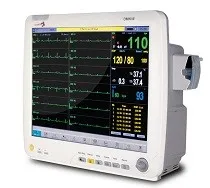
Infinium is pleased to announce that the FDA 510K has been granted for our new 12 lead ECG and Cardiac Output functions on the Omni series patient monitors. Omni III patient monitors with 12 lead ECG and Cardiac Output are in stock and available for immediate installation in all FDA governed jurisdictions as well as globally. Should you need a copy of the latest FDA 510K certificate, please e-mail your request to: sales@site_99d86bc1-9f52-4353-b1cf-4e5e15333b21
Please download the latest product literature here.

