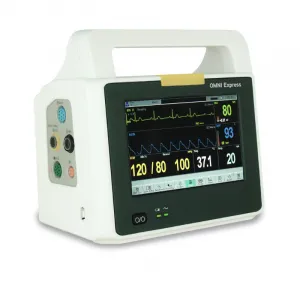
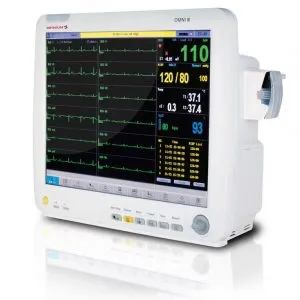
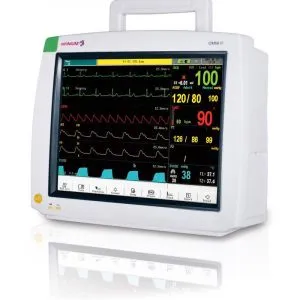
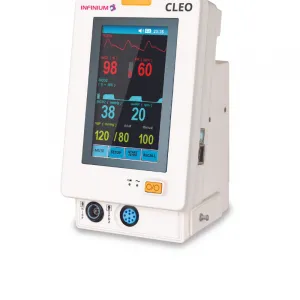
Infinium Medical, Inc., a US Manufacturer of medical devices, is proud to announce receipt of the European Union Medical Device Regulation (EU MDR) Certification for OMNI Series and CLEO Patient Monitors.
The devices had been previously cleared under the now superseded Medical Device Directive (MDD) and have now successfully achieved this new certification, navigating the new, more stringent requirements set forth in the EU MDR.
“This transition has been a challenge for the medical device industry, and we are extremely delighted and proud of our team for this significant achievement. In receiving this certification, Infinium has demonstrated our proven commitment to quality and ability to meet the heightened requirements put forth by the European Union for the safety and effectiveness of medical devices,” said Nick Wilkins, Director of Quality Assurance and Regulatory Affairs.
With this clearance, Infinium is now able to again affix the CE mark to their line of patient monitors and continue to serve patients and partners in Europe and around the globe.
CE Certified Omni & Cleo Patient Monitors
Why MDR Matters
Under the old Medical Devices Directive (MDD), each EU member state took the Directive’s provisions and transposed them into national law, creating a patchwork of sometimes slightly varying requirements. The new Medical Device Regulation (MDR) is directly applicable in all EU member states, which makes it more uniform—but also more stringent in its demands. From a quality process perspective, the MDR has several key differences from the MDD:
- Stricter Clinical Evidence Requirements
- MDR places a heavier emphasis on clinical evaluation and real-world clinical evidence. Manufacturers must show more robust data to prove safety and performance, often requiring stronger clinical studies or post-market clinical follow-up.
- Expanded Scope & Classification Rules
- Certain products that previously fell outside the MDD scope are now included under MDR (e.g., some cosmetic or aesthetic devices).
- Revised classification rules (e.g., for software) can push devices into higher risk classes, thus requiring more in-depth conformity assessments.
- Enhanced Post-Market Surveillance (PMS) and Vigilance
- MDR mandates a more proactive PMS system, including continued gathering of clinical and performance data from the field.
- Risk management and clinical follow-up activities are expected to be ongoing throughout the product’s lifecycle, rather than limited to initial product development.
- Increased Transparency and Traceability
- MDR introduces the Unique Device Identification (UDI) system for better traceability of devices.
- The EUDAMED database will offer more transparency around device registration, certification status, and safety information.
- Greater Responsibility for Economic Operators
- Manufacturers, importers, distributors, and Authorized Representatives each have more clearly delineated obligations—especially regarding quality management, recordkeeping, and incident reporting.
Manufacturers, importers, distributors, and Authorized Representatives each have more clearly delineated obligations—especially regarding quality management, recordkeeping, and incident reporting.
In practical terms, transitioning from MDD to MDR means an updated quality management system (QMS) to meet these heightened requirements. These changes, while more demanding, aim to ensure higher safety and performance standards for devices placed in the market.
Why is it related to European CE Certification?
The Medical Device Regulation (MDR) effectively replaced the Medical Device Directive (MDD) for CE-marking medical devices in the EU. Although there was a transition period, MDR is now the primary path for new CE certifications. Here’s how it fits together:
- MDR Replaced MDD
- The MDR entered into full application on May 26, 2021, replacing the MDD (93/42/EEC) and the Active Implantable Medical Devices Directive (90/385/EEC).
- New or significantly changed devices must comply with MDR requirements to obtain (or renew) a CE mark.
- Transitional Arrangements
- Certain MDD or AIMDD certificates (issued prior to MDR’s application date) may remain valid until their expiry date, provided the devices and certificates meet specific transition criteria.
- Depending on the device risk class and certificate date, extensions may apply, but new certifications now follow MDR rules.
- Role in CE Certification
- CE Marking is the overall conformity mark indicating that a product meets all relevant EU requirements. For medical devices, this means meeting the Essential Requirements under MDD in the past, now replaced by the more rigorous General Safety and Performance Requirements (GSPRs) under MDR.
- If you are placing a new medical device on the EU market, your notified body assessment and CE certification must be under MDR (unless you’re covered by a valid, unexpired MDD certificate during the transition period).
- Impact on Quality Management System (QMS)
- Achieving CE certification under MDR generally requires demonstrating compliance with ISO 13485 or an equivalent QMS, plus robust processes for clinical data, post-market surveillance, and vigilance—all spelled out in MDR.
- Many companies have had to update their QMS to ensure it aligns with stricter MDR demands, such as continuous clinical evaluation and improved traceability (UDI system).
In short, MDR is now the main regulatory framework you must follow to obtain (or maintain) CE certification for medical devices in the EU. Any legacy MDD certificates will eventually expire, and from that point on, only devices compliant with the MDR can lawfully bear the CE mark for EU market access.